 |
| The three forms of trypansomes - slender, intermediate and stumpy. |
Without drugs, it is impossible for an infected person to deal with the infection and the disease is always fatal. But sleeping sickness has a fairly high rate of relapse even after treatment. One of the reasons for this could be that, at some point the parasite decides to make the trip from the blood and into the brain. Here, it is effectively protected from drug treatment, and can pass back into the blood system to continue the infection. An evolutionary explanation for this could be that some hosts are better at dealing with the infection than humans, and the brain represents a hiding place from the immune system.
 |
| Tsetse fly. Yuk. Image from Wikipedia |
But how does the trypanosome succeed in altering a person’s sleeping patterns? It appears that this is a side effect of a signalling molecule used by trypanosomes to control cell density. When the parasite gets into the brain, it doesn’t want to cause extensive inflammation and get itself noticed. So it secretes a messaging molecule called PGD2 that tells neighbouring parasites to commit parasite-suicide for the good of the overall population. But PGD2 has also been shown to cause non-REM sleep when injected into the nervous system. So secreting PGD2 directly into the brain is useful to the parasite when a person is far more likely to be bitten by the tsetse fly if they fall asleep during the day.
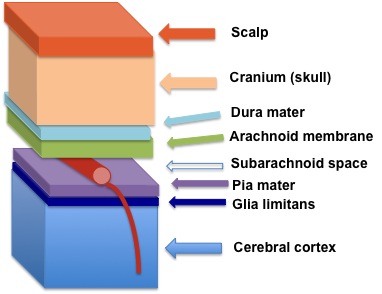 |
| The sleeping sickness parasite makes its way to reside between the Pia mater and Glia limitans at the edge of the blood. Image from: Wikipedia |
But the group responsible for this work also addressed the question of why the brain stage takes so long to emerge. Something interesting about their attempts to reproduce the brain infection in rats was that it proved impossible to simply inject parasites into the nervous system. Instead, the infection needed to take its usual course, beginning with the blood stage and progressed to the brain stage after some time. It appears that there are three forms of the parasite (shown in the figure at the top of the post)—a stumpy form which does not undergo the variation in its coat proteins and is killed by the immune system, an intermediate form which is responsible for the blood infection, and a slender form which can cross into the brain. How this slender form emerges and whether it really is required for brain infection remains to be determined, however.
Research such as this has the potential to help the development of future vaccines and drugs by teaching us more about how the infection progresses. The current treatment for the later brain stage of the disease involves an arsenic-derivative which kills one in twenty people and has been described as ‘fire in the veins’ by those unlucky enough to need to take it. Over the past few years, sleeping sickness has slowly been decreasing in numbers and it is hoped that in a decade this disease may finally be eliminated.












No comments:
Post a Comment